公司信息
北京思齐生物技术有限公司
电话:400-108- 7060
010-82250780
Q Q:345747867
1244976716
67965156
网址:www.skillbio.com
邮箱:info#skillbio.com
产品介绍
技术参数
资料下载
浏览:0 次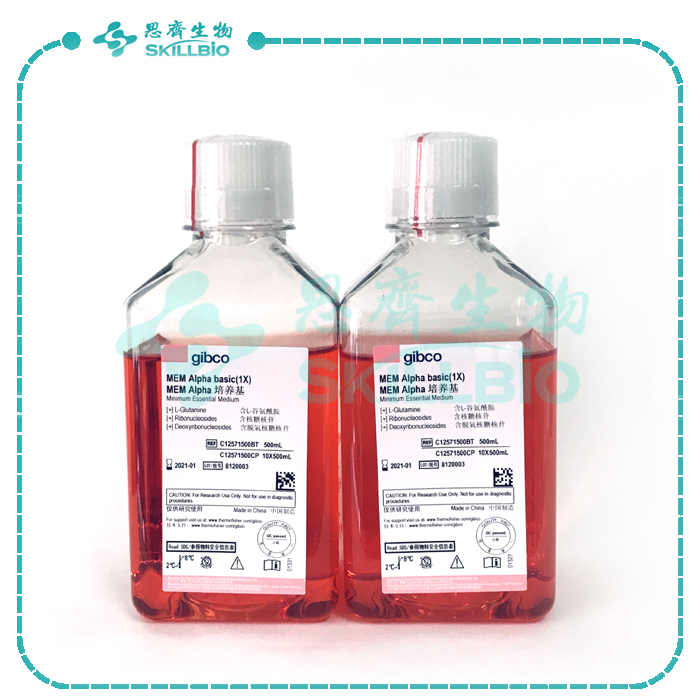


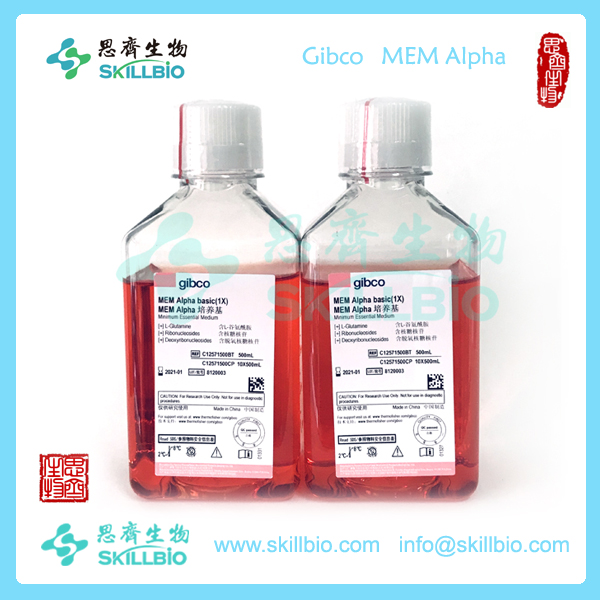
Minimum Essential Medium (MEM) α is widely used for mammalian cell culture as well as selection for transfected DHFR negative cells. MEM α can be used with a variety of suspension and adherent mammalian cells, including keratinocytes, primary rat astrocytes, and human melanoma cells. Life Technologies offers a variety of Gibco® MEM α modifications for a range of cell culture applications. Find the right formulation using the media selector tool.
This MEM α is modified as follows:
| With |
| • Ribonucleosides |
| • Deoxyribonucleosides |
| • L-glutamine |
| • Phenol Red |
The complete formulation is available.
Gibco® MEM α is a modification of Minimum Essential Medium (MEM) that contains non-essential amino acids, sodium pyruvate, lipoic acid, B12, biotin, and ascorbic acid. MEM α is available without nucleosides for use as a selection medium for DG44 and other DHFR negative cells. This product is made with Earle’s salts.
Product Intended Use
For in vitro diagnostic use. CAUTION: Not for human or animal therapeutic use. Uses other than the intended use may be a violation of local law.
cGMP Manufacturing and Quality System
Gibco® MEM α is manufactured at a cGMP compliant facility, located in Paisley, Scotland, UK. The facility is registered with the FDA as a medical device manufacturer and is certified to the ISO 13485 standard. For supply chain continuity, Life Technologies offers an identical Gibco® MEM α product made in our Grand Island facility (12571-063). This facility is registered with the FDA as a medical device manufacturer and is certified to the ISO 13485 and ISO 9001 standards.
MEM α contains no proteins, lipids, or growth factors. Therefore, MEM α requires supplementation, commonly with 10% Fetal Bovine Serum (FBS). MEM α uses a sodium bicarbonate buffer system (2.2 g / L) and therefore requires a 5-10% CO2 environment to maintain physiological pH.